References: hydrogen.ecn.purdue.edu & news.uns.purdue.edu
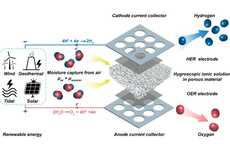
A new aluminum rich alloy has been developed by Purdue University Engineers that could solve the main problem preventing the widespread use of hydrogen-powered vehicles. That is, hydrogen can be readily produced by a number of methods, but safely and efficiently transporting it, distributing it, and storing it aboard automobiles has proven to be the biggest hurdle. Enter the new alloy from Purdue, made of 95 percent aluminum, and 5 percent a second alloy of gallium, indium and tin. When submerged in water, the gallium/indium/tin alloy acts as a catalyst and allows the aluminum to react with the water, a characteristic it normally doesn't possess. This reaction produces pure hydrogen and leaves behind aluminum oxide, which can be efficiently recycled back into aluminum. The catalyst alloy is inert, allowing it to also be recycled. This advance opens up the possibility of refueling a vehicle with recyclable cartridges containing the new alloy, and water. If the hydrogen is used to power a fuel cell, the only gas that would be emitted during operation of the vehicle would be water vapor.
This material is a significant improvement over previous materials, also developed at Purdue, that required the gallium/indium/tin alloy to be in a liquid phase, and also uses less of the expensive gallium. The research team will be presenting their latest findings at the Materials Innovations in an Emerging Hydrogen Economy conference, on Feb. 26 in Cocoa Beach, Fla..
This material is a significant improvement over previous materials, also developed at Purdue, that required the gallium/indium/tin alloy to be in a liquid phase, and also uses less of the expensive gallium. The research team will be presenting their latest findings at the Materials Innovations in an Emerging Hydrogen Economy conference, on Feb. 26 in Cocoa Beach, Fla..
Trend Themes
1. Hydrogen Storage Solutions - The development of the aluminum rich alloy provides a breakthrough in safe and efficient hydrogen storage, opening up opportunities for hydrogen-powered vehicles.
2. Recyclable Energy Cartridges - The new alloy enables the possibility of refueling vehicles with recyclable cartridges, reducing the need for traditional fueling infrastructure.
3. Sustainable Fuel Cells - The use of hydrogen as a fuel source in fuel cells, powered by the newly developed alloy, could lead to the emission of only water vapor during vehicle operation, creating a sustainable energy alternative.
Industry Implications
1. Automotive - The automotive industry can explore opportunities in hydrogen-powered vehicles, utilizing the aluminum rich alloy for safe and efficient hydrogen storage.
2. Renewable Energy - The renewable energy sector can benefit from the development of recyclable energy cartridges, providing a more sustainable and convenient storage solution for hydrogen.
3. Materials Science - The materials science industry can leverage the advancements in developing sustainable fuel cells, using the new alloy as a catalyst for hydrogen reactions and reducing emissions.
2.6
Score
Popularity
Activity
Freshness